The Scientific Research Team of the State Key Laboratory of Respiratory Disease Confirmed that the Combination of the New Drug TB47 and the Old Drug is Expected to Cure MDR-TB in 5 ...
2020-09-281505Leader of the tuberculosis team of the State Key Laboratory of Respiratory Disease, Tianyu Zhang’s research team, provided the latest research results and confirmed that the laboratory’s new anti-tuberculosis drug TB47, which targets the mycobacterial respiratory chain cytochrome bc1 oxidase complex (transferred and entered the GLP safety evaluation stage) and Clofazimine have a unique characteristic of bactericidal and sterilizing activity. The results were published online on Biomedicine & Pharmacotherapy on September 25th. The co-first authors are doctoral student Wei Yu and international doctoral student Gift Chiwala.
The research team used the unique non-resistant marker autoluminescent mycobacterium tuberculosis at the bacterial level and macrophage infection level to prove that TB47 and Clofazimine can form a unique synergistic bactericidal effect. In the end, a parallel comparative experiment with Bengali Therapy in an animal model showed that 43.75% of animals (7/16) were cured after 3 months of treatment with TB47 and 84.2% (16/19) ~87.5% (14/16) were cured after 4 months of treatment, and no recurrence occurred after 5 months of treatment (20 mice in the first animal testing, 16 mice in the second). The results of the Bengali Therapy as a control group is nearly identical to the results previously published by Johns Hopkins University in the United States. As a result, we infer that the Bengali Therapy with the addition of TB47 may shorten the previous course of treatment of MDR-TB from more than 9 months to less than 5 months, which is nearly the same with the 6-month course of treatment currently needed for common tuberculosis with four first-line drugs.
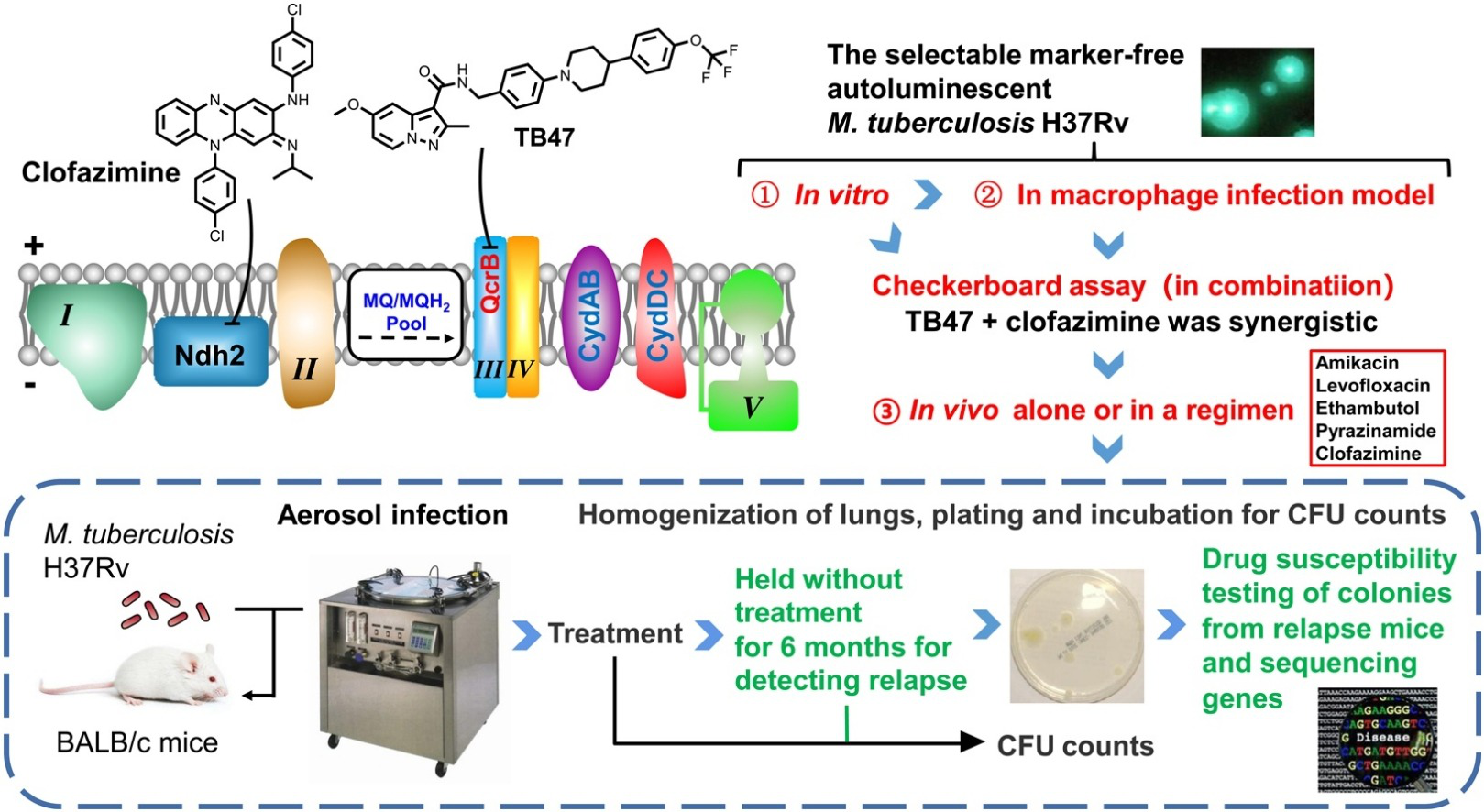
Although it was confirmed in 2019 that the new drugs bedaquiline + putomani + linezolid can cure MDR-TB in 6.5 months, their toxicity is very high and the price is extremely expensive, not to mention that some people voted against the treatment in the USFDA Drug Review. In recent years, some of China’s new anti-TB drugs have completed Phase I of clinical trials. The combination of these new drugs + old drugs may become the “Chinese therapy” that accelerates the cure for MDR-TB, allowing China, which has long been the second country in the world with the highest burden of TB, to make a contribution to the R&D in the field of TB prevention and control.
This research was supported by the National Science and Technology Major Project and the Major New Drug Development Project and the Chinese Academy of Sciences. Meanwhile, we would like to show our gratitude to Guangdong Special Support Program “Science and Technology Leading Talents” for supporting Tianyu Zhang, as well as the CAS-TWAS President’s Fellowship Program and the International Scholarship of Chinese Academy of Sciences Fellowship Program (PIFI) for supporting international students and scholars.
















