The Latest Meta-Analysis Is More Than Synergies: Combination Chemotherapy Can Reduce the Immunotoxicity of PD-1/L1 Monoclonal Antibody
2020-11-041445Immune checkpoint PD-1 and PD-L1 inhibitors have completely changed the treatment pattern for non-small cell lung cancer (NSCLC). Multiple studies have shown that PD-1/L1 inhibitors can significantly improve progression-free survival (PFS) and overall survival (OS) in patients with advanced NSCLC, whether it is first-line or later-line treatment. In addition, compared with chemotherapy, the PD-1/L1 inhibitors are saver, which has also been confirmed in different clinical trials. However, PD-1/L1 inhibitors can cause immune-related adverse events (irAE) due to imbalance in the immune system, for example, immune-related pneumonia and hepatitis, which have become a major problem for clinicians, as they can lead to serious consequences, such as the interruption of treatment and even death.

The research team led by Professor He Jianxing and Liang Wenhua of the State Key Laboratory of Respiratory Disease recently published a Meta-analysis paper on the indirect comparisons for single-arm trials in the international Journal Cancer, which compares the influence of combination chemotherapy on irAE based on immune checkpoint inhibitors. The first authors of the paper are Wang Manting, Liang Hengrui and Wang Wei.
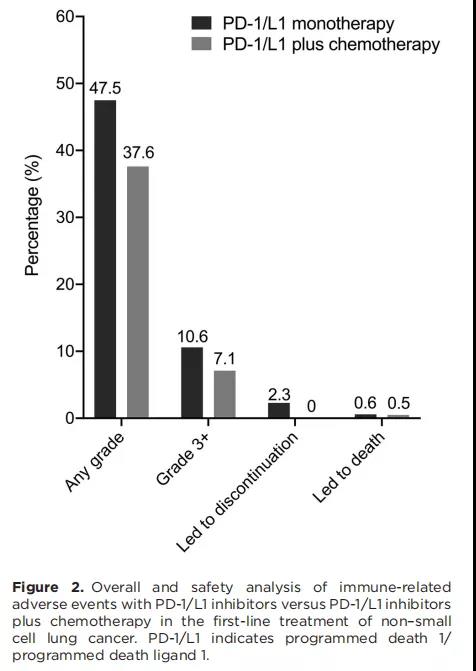
The research has studied a total of 5,931 patients in 10 studies. The research points out that generally speaking, when PD-1/L1 is combined with chemotherapy, the incidence of irAE above grade 3 is lower than that of single-agent PD-1/L1 (7.1% vs 10.6%; RR: 0.516, 0.291-0.916).
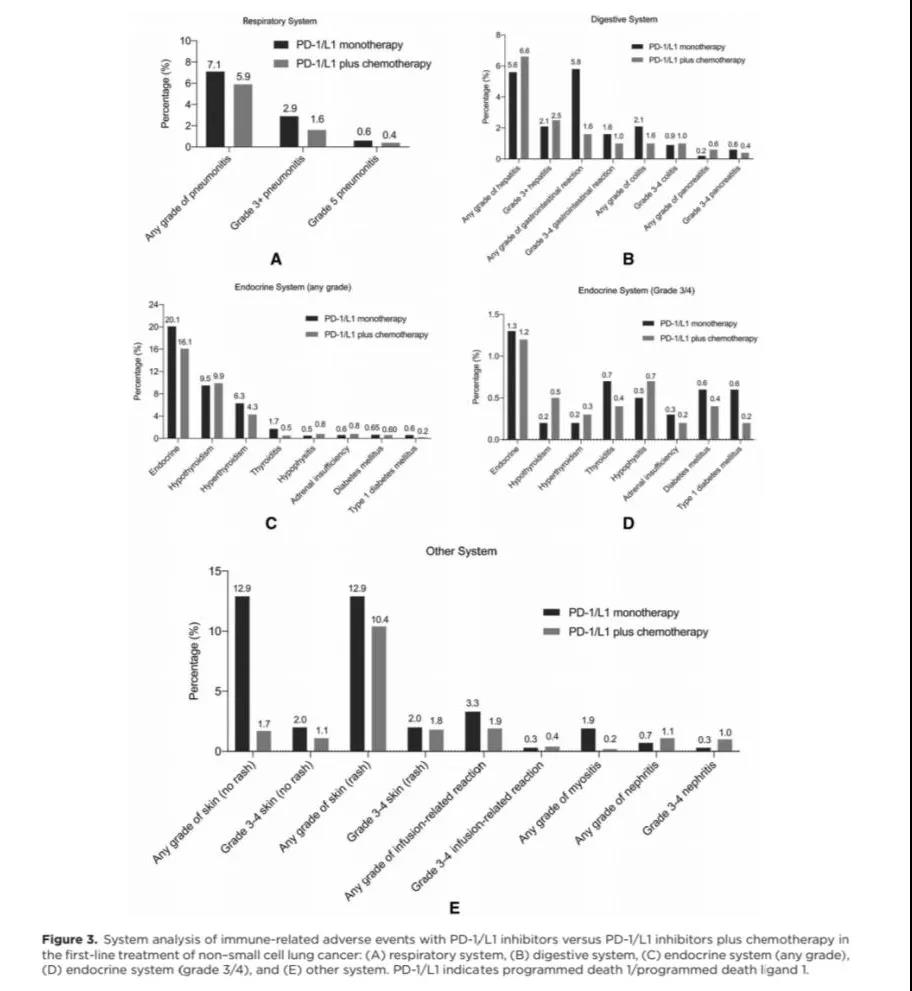
Subgroup analysis of different systems suggests that compared with single agent PD-1/L1, in the combination of PD-1/L1 and chemotherapy, the incidence of irAE is lower, such as immune-related pneumonia (5.9% vs 7.1%; RR: 0.217, 0.080-0.588), immune-related endocrine-related adverse events (16.1% vs 20.1%; RR: 0.260, 0.120-0.564) and immune-related skin adverse events (10.4% vs 12.9%; RR: 0.474, 0.299-0.751). The immune-related adverse events related to the digestive system were similar in the two groups.
Conclusion: the research has pointed out that during the first-line treatment of NSCLC, compared with the single agent PD-1/L1 inhibitor, the combination of chemotherapy and PD-1/L1 inhibitor can reduce the overall and the incidence of most irAE, for example, the irAE of immune-related pneumonia, immune-related endocrine-related adverse events and immune-related skin adverse events.
















