Professor Zhang Tianyu’s Team from the Lab Proved BCG can Assist in the Treatment of XDR-TB
2021-08-24974Tuberculosis is a fatal disease caused by Mycobacterium tuberculosis (TB for short), and pulmonary tuberculosis is the most common. TB has become the world’s leading infectious disease since 2014 (except for COVID-19). With the continuous emergence of extensively drug-resistant tuberculosis (XDR-TB), tuberculosis reappears. Zhang Tianyu’s team at the Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences\State Key Laboratory of Respiratory Diseases has spent nearly 7 years developing a therapeutic live vaccine, which has been proven to assist in the treatment of XDR-TB through animal testing. The results were published online in journal Biomedicine and Pharmacotherapy on August 18.
The vaccine which is called RdrBCG (recombinant drug-resistant BCG) was developed based on BCG. BCG, namely, Mycobacterium bovis bacille Calmette Guerin, is an attenuated mycobacterium bovis and is currently the only preventive tuberculosis vaccine used in clinical practice. It is very similar to the human mycobacterium tuberculosis, and their genetic sequence similarity is over 99%. Can BCG assist in the treatment of tuberculosis? No, it can’t in theory, because almost all drugs for treating tuberculosis are effective on BCG, and BCG may have been killed by anti-tuberculosis drugs before it works. Therefore, they proposed the preparation of selective drug-resistant BCG, that is, to enable the prepared BCG to resist the drugs selected for the treatment of tuberculosis. In addition, some recombinant BCG showed an enhanced effect on the immune protection from tuberculosis after certain gene overexpression. As a result, researchers selected two proteins, Ag85B and Rv2608, which show higher-level expression in the active and dormant states, respectively, and it is reported that they have good immune protection effects. Ag85B enhanced the survival of BCG in its host, the macrophages, and stimulated the expression of protective immunity, while Rv2628 stimulated the production of protective immune cells and helped BCG overcome stress response to critical events in the host. They integrated these two genes into the prepared genome of drug-resistant wild-type BCG, to obtain a new recombinant drug-resistant BCG (RdrBCG-I) in which these two foreign genes can be expressed very stably in vivo and in vitro.
Animal testing shows that both RdrBCG-I and wild BCG show strong safety in mice with severe combined immunodeficiency. Even if infected with a large amount of BCG, no mouse died or even got sick during the 5-month observation period; RdrBCG-I only needed 3 immunizations to continue sterilization, which cannot be achieved by general drugs (combination) in the consolidation treatment. The immunization of RdrBCG is achieved through subcutaneous injections or airway, and their effect is similar. The adjuvant treatment of RdrBCG-I in drug-resistant tuberculosis can also improve pulmonary disease, which is expected to improve the effect of rehabilitation after recovery. The results also proved that the effect is the same with or without ordinary BCG in existing therapies, which means ordinary BCG cannot be used for adjuvant therapy.
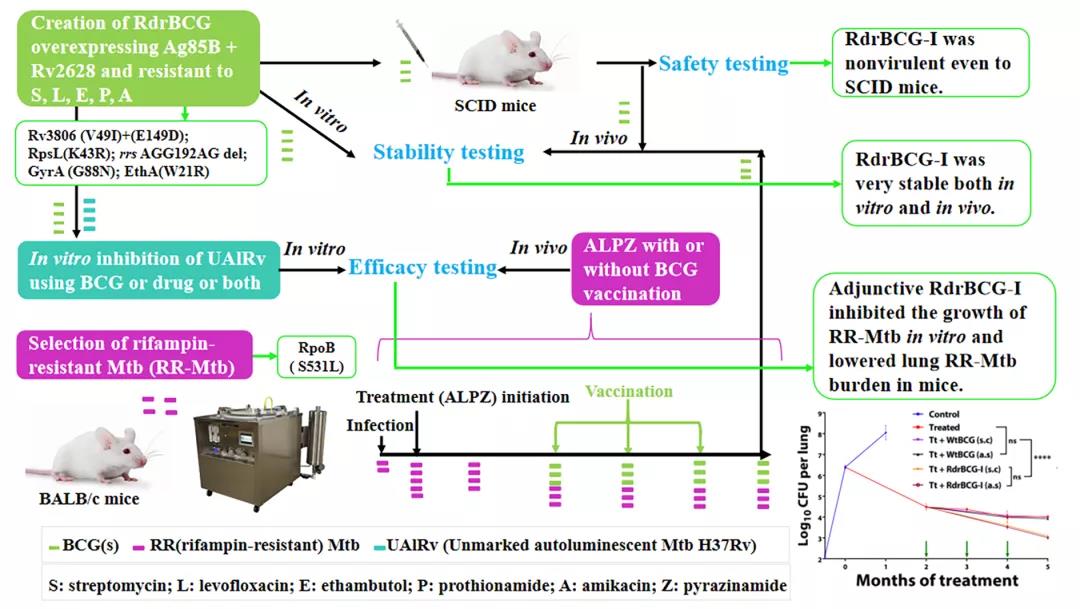
RdrBCG provides a new concept for the design of therapeutic vaccines against refractory pathogens like mycobacterium tuberculosis. The research is the result of interdisciplinary research in immunology, pathology, microbiology, pharmacology, microecology, and molecular biology. At present, it has applied for the national invention patent and international patent.
Original paper:https://www.sciencedirect.com/science/article/pii/S0753332221008301?via%3Dihub
















