The team led by Liu Jinsong of the laboratory reveals the mechanism of symmetric glycosylation in Elaiophylin biosynthesis
2022-10-241426Recently, the teams led by deputy director Liu Jinsong and Zhang Changsheng of the laboratory worked together to interpret the mechanism of ElaGT symmetric glycosylation. The results were published entitled “Substrate-induced dimerization of elaiophylin glycosyltransferase reveals a novel self-activating form of glycosyltransferase for symmetric glycosylation” in the International Crystal Society’s journal Acta Cryst. On D Struct Biol (IF: 5.699).
Elaiophylin is a natural macrolide antibiotic with C2 symmetry, with a deoxy glycosyl at each end. The antibiotic is novel in structure, with a broad spectrum of antibacterial activity, and has strong antimicrobial activity against various drug-resistant bacteria such as methicillin-resistant staphylococcus aureus, and multi-drug resistant and widely drug-resistant Mycobacterium tuberculosis. ElaGT (elaiophylin glycosyltransferase) is the enzyme for the symmetrical transfer of elaiophylin glycosyl cloned by Zhang Changsheng of the South China Sea Institute of Oceanography, Chinese Academy of Sciences. It is a potential enzymatic tool, but there is no report on the catalytic mechanism of such symmetric glycosylation.
By analyzing the structure of ElaGT in four states, it has revealed that ElaGT can form a dimer with a continuous channel when induced by substrate whose length allows an Ela molecule to shuttle within the channel. Through further structural analysis and biochemical experiment, it was revealed that this dimer was assembled in a similar manner to the mode of action of enzyme and ligand in activator-dependent glycosyltransferase, where the dimerization interface can indirectly regulate the glycosyl-binding site. Therefore, the enzyme may adopt a self-activated dimerization mode to identify and catalyze the glycosyl transfer, and the interface may be a potential regulatory site of enzyme activity and substrate specificity. This study provides new ideas for the synthesis of novel analogues with better antimicrobial activity.
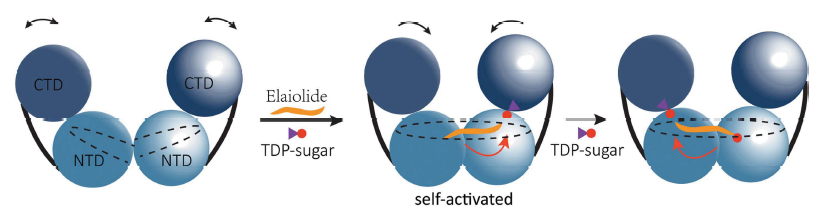
When ElaGT binds with the substrate, a dimer with a continuous channel is formed, allowing an Ela molecule to shuttle inside
Original paper:https://onlinelibrary.wiley.com/doi/abs/10.1107/S2059798322008658?sentby=iucr
















