PNAS: The Team Led by Professor Zhao Jincun Successfully Established Mouse Models Susceptible to HCoV-229E and HCoV-NL63 and Elucidated Cross-immune Protection against SARS-CoV-2 I...
2023-01-211573Human coronavirus HCoV-229E and HCoV-NL63 are seasonal common cold virus, which can prevail worldwide and mainly infect the upper respiratory tract of humans and cause common cold symptoms (1-2). However, among the populations with low immunity, such as children, the elderly and patients with immune deficiency, it can cause severe pneumonia and bronchitis and even lead to death (3-6). After getting infected by 229E, minority healthy adults may develop acute respiratory distress syndrome (7). According to the earlier research conducted by our team, NL63 is now evolving actively, whose new sub-type may cause severe pneumonia among children (8). However, since there is no mouse model of 229E and NL63, there are no special drugs and vaccines for the infection of 229E and NL63. It is still unclear whether they have cross body fluid and cellular immune response with the current pandemic of SARS-CoV-2.
On January 18, the team led by professor Zhao Jincun, the First Affiliated Hospital of Guangzhou Medical University/SKLRD worked with professor Malik Peiris of Hong Kong University and the State Key Bio-safety Testing Laboratory of Guangzhou Customs Technology Center (P3 Laboratory) to publish on the journal of PNAS a research paper with the title of “Mouse models susceptible to HCoV-229E and HCoV-NL63 and cross protection from challenge with SARS-CoV-2”. The author used adenovirus vector expressing the receptor of 229E and NL63 and injected into the mice with different genetic backgrounds with nasal drip so as to quickly establish mouse models susceptible to 229E and NL63. The model can be applied in the evaluation of the broad-spectrum antiviral drugs and vaccines, and clarify that there is cross protection between the 229E, NL63 and novel coronaviruses in vivo, thus providing a platform for the research on the pathogenic mechanism of coronavirus.

The receptor of 229E is aminopeptidase N (APN) (9), and the receptor of NL63, SARS-CoV and SARS-CoV-2 is angiotensin-conveting enzyme 2 (ACE2) (10-14). In this research, the research team used the adenovirus vector to respectively express the receptor hAPN of 229E and the receptor hACE2 of NL63 in the lungs of IFNAR-/- and STAT1-/- mice, which were infected by 229E and NL63 five days after the transduction. After the infection, interstitial pneumonia appeared in the lungs of mice, and the virus replication can persist for about 7 days. Virus-specific T cell response and neutralizing antibodies can be generated in the mice susceptible to 229E and NL63. Treated with Remdesivir in the mice infection model, it can accelerate the clearing of virus. Using the VEE Replicon Particle (VRP) respectively expressing 229E-S or NL63-S to immunize the mice, it can also accelerate the clearing of virus. It indicates that the established mouse models susceptible to 229E and NL63 can be used in the evaluation of drugs and vaccines.
Further, in order to find out whether 229E and NL63 have cross immune protection of the highly pathogenic SARS-CoV-2, the research team used 229E and NL63 to infect the mice and made the mice infected by SARS-CoV-2 during the infection memory period. The results showed that compared with the control group, pre-infection by 229E and NL63 can reduce the body weight loss of mice and accelerate the clearing of virus and ease the pathological damage of the lungs. Later, during the recovery period from the infection of 229E and NL63, the serum had no protection. Before infection by SARS-CoV-2, delete antibodies were used to delete the CD4+ and CD8+T cells, which can delay the clearing of SARS-CoV-2. This implies that the cross immune protection may be mediated by the cross responsive T cells (see Fig. 1).
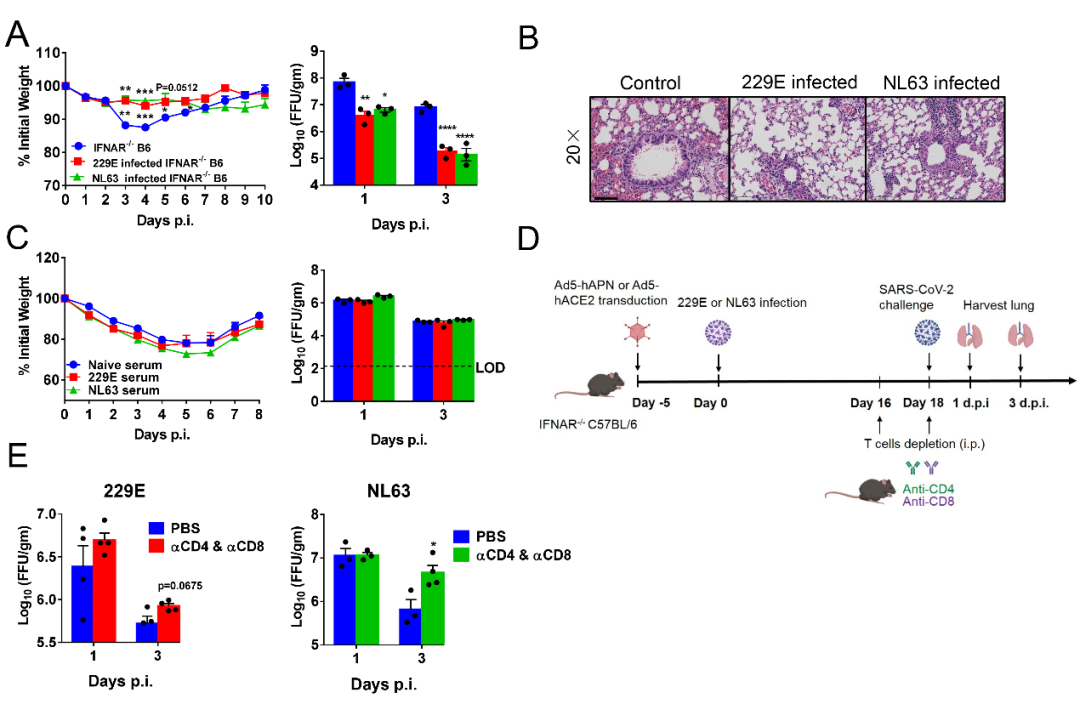
Fig. 1 Mice susceptible to 229E and NL63 can partially resist to the infection of SARS-CoV-2
Professors Zhao Jincun and Zhao Jingxian with the SKLRD and professor Malik Peiris with Hong Kong University are the co-corresponding authors of the paper, while the co-first authors include Dr. Liu Donglan, Chen Chunke, Chne Dingbin, Zhu Airu, Li Fang, Zhuang Zhen with the First Affiliated Hospital of Guangzhou Medical University/SKLRD, professor Chris Ka Pun Mok with Chinese University of Hong Kong, Dr. Dai Jun and Li Xiaobo with the State Key Bio-safety Testing Laboratory of Guangzhou Customs Technology Center (P3 Laboratory) and Dr. Jin Yingkang with Guangzhou Women and Children Medical Center. The research has been funded by the Ministry of Science and Technology and the National Natural Science Foundation, etc.
References
1. van der Hoek, L., Human coronaviruses: what do they cause? Antivir Ther, 2007. 12(4 Pt B): p. 651-8.
2. Davis, B.M., et al., Human coronaviruses and other respiratory infections in young adults on a university campus: Prevalence, symptoms, and shedding. Influenza Other Respir Viruses, 2018. 12(5): p. 582-590.
3. Pene, F., et al., Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis, 2003. 37(7): p. 929-32.
4. Walsh, E.E., J.H. Shin, and A.R. Falsey, Clinical impact of human coronaviruses 229E and OC43 infection in diverse adult populations. J Infect Dis, 2013. 208(10): p. 1634-42.
5. Gorse, G.J., et al., Human coronavirus and acute respiratory illness in older adults with chronic obstructive pulmonary disease. J Infect Dis, 2009. 199(6): p. 847-57.
6. Cui, J., F. Li, and Z.L. Shi, Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol, 2019. 17(3): p. 181-192.
7. Vassilara, F., et al., A Rare Case of Human Coronavirus 229E Associated with Acute Respiratory Distress Syndrome in a Healthy Adult. Case Rep Infect Dis, 2018. 2018: p. 6796839.
8. Wang, Y., et al., Discovery of a subgenotype of human coronavirus NL63 associated with severe lower respiratory tract infection in China, 2018. Emerg Microbes Infect, 2020. 9(1): p. 246-255.
9. Yeager, C.L., et al., Human aminopeptidase N is a receptor for human coronavirus 229E. Nature, 1992. 357(6377): p. 420-2.
10. Li, W., et al., The S proteins of human coronavirus NL63 and severe acute respiratory syndrome coronavirus bind overlapping regions of ACE2. Virology, 2007. 367(2): p. 367-74.
11. Zhou, P., et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 2020. 579(7798): p. 270-273.
12. Li, W., et al., Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature, 2003. 426(6965): p. 450-4.
13. Hoffmann, M., et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, 2020.
14. Hofmann, H., et al., Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc Natl Acad Sci U S A, 2005. 102(22): p. 7988-93.
Original paper:https://www.pnas.org/doi/10.1073/pnas.2202820120
















