The Team Led by Professor Su Jin Participates in Development of New Radioactive Drugs for Early Diagnosis of Fibrosis Diseases
2021-11-051091Recently, the research team led by Su Jin of the SKLRD worked with the team led by Professor Tang Ganghua of the Department of Nuclear Medicine, Nanfang Hospital to achieve a significant headway in the early detection of pulmonary fibrosis, whose related research findings were published on the Acta Pharmaceutica Sinica B (Zone 1 of Chinese Academy of Sciences, IF=11.413) with the title of “Preclinical evaluation and pilot clinical study of [18F]AlF-labeled FAPI-tracer for PET imaging of cancer associated fibroblasts”, and the new PET probe was granted with an invention patent of China (202011085122.7).
The researchers have designed a new tracer of Fibroblast activation protein (FAP), conducted pre-clinical in vitro and in vivo comparison with other FAP tracers reported abroad, and performed PET/CT imaging on one Nasopharyngeal cancer patient. The results indicated that chemical restructuring had increased the uptake value and detention time of the tracer at the focus, and clearly showed the primary tumor and lymphatic matastasis of the aforesaid patient.
The research paper’s first author is Hu Kongzhen, an associate research fellow with Nanfang Hospital of Southern Medical University and its corresponding author is Tang Ganghua, a research fellow with Nanfang Hospital of Southern Medical University and Professor Su Jin with SKLRD.
Research background and significance
According to authoritative statistical data, 45% of human’s diseases and deaths are related with organ fibrosis. Fibrosis may occur to any organ due to injuries. Tumor is called a wound that never heals, which also belongs to the category of fibrosis diseases in the broad sense. The common characteristics of various organ fibrosis cases at the level of cellular elements are the excessive activation of fibrosis cells and the excessive sedimentation of extracellular matrix.
Due to the lack of noninvasive, sensitive biomarkers, fibrosis diseases usually develop to the irreversible middle and advanced stages when they are diagnosed, which is considered as the main cause why the idiopathic pulmonary fibrosis (IPF) patients can survive for a period of only three years on average after they are diagnosed. Though currently two anti-fibrosis drugs have been approved clinically, the judgement of the time to use the drug and the evaluation of therapeutic effect have always been the “bottlenecks” in this area.
Fibroblast activation protein (FAP) is a membrane protein with activity of serine protease, which has been proved as activated fibroblasts with high expression under multiple diseases, but cannot be expressed in normal tissues of human body. After the failure of FAP compounds being used in drug development, the conjugated positron nuclide 68Ga discovered by the nuclear chemical experts of University of Heidelberg, Germany in 2019 was successfully used in the imaging of 28 kinds of human tumors. The breakthrough had been selected among the annual progresses of nuclear medicine in 2019.
Therefore, further developing and optimizing the FAP tracers marked with nuclides is vital to the diagnosis and treatment of fibrosis diseases in the broad sense. The several kinds of FAP tracers marked with 68Ga or 18F reported in recent years (Fig. 1) are of imaging values to the FAP related diseases, yet they all have problems such as quickness to be cleared in the body, and a short detention time in the focus of infection. The new FAP tracer (18F) AlF-P-FAPI developed in this research boasts the advantages such as a high uptake value in the focus, a long detention time and in vivo stability, simple for automatic production, with a high radiochemical yield. The initial clinical tests have proved that it is safe and feasible in human body imaging. Therefore, [18F]AlF-P-FAPI is a promising nuclide photographic developer, which has the value for further expanding the researches of clinical indications.
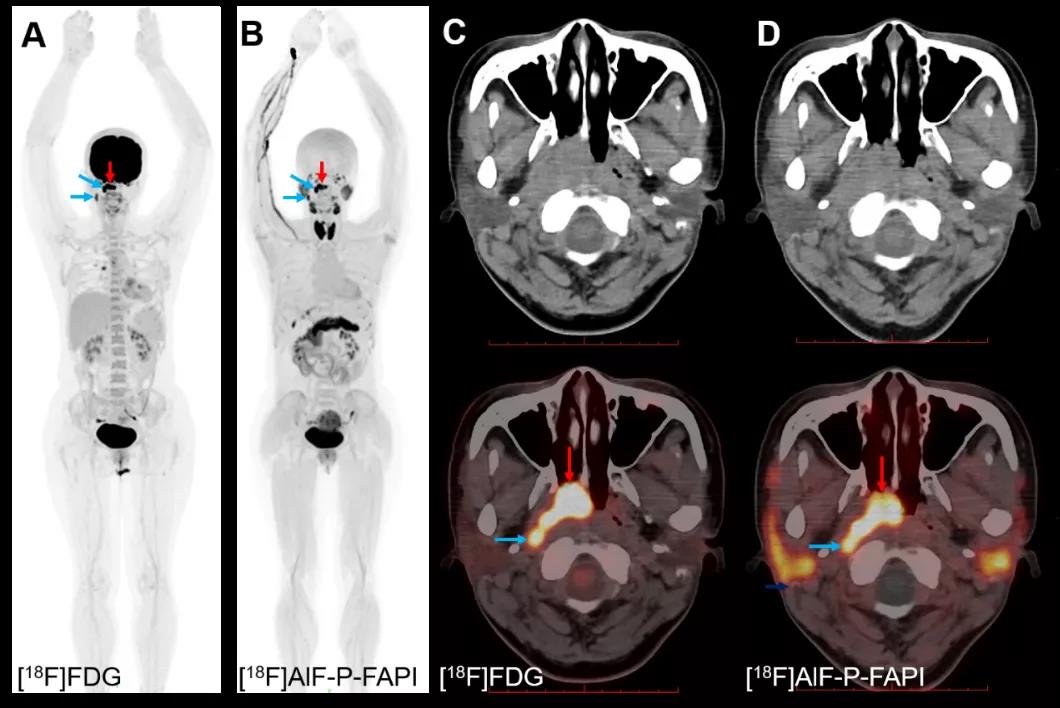
PET/CT imaging of nasopharyngeal cancer patient with [18F]AlF-P-FAPI and [18F]FDG
Original paper:https://www.sciencedirect.com/science/article/pii/S2211383521003932?v=s5
















