The Random, Double-blind and Control Study on the Effectiveness and Safety of Using Lung Tonifying and Blood Circulation Promoting Capsule to Treat Covid-19 Patients under Rehabilit...
2021-11-161252
At present, the Covid-19 is still rampant around the world. As the total number of Covid-19 patients continues to increase worldwide, the rehabilitation problem of the patients is increasingly prominent. It has been found in a number of clinical follow-up studies that the vast majority of the patients discharged from hospital still have abnormal symptoms such as fatigue and sleep disorder one year after they got infected by the virus. In August 2021, the journal “Lancet” published an editorial to call for attention to the rehabilitation period of the Covid-19 patients and propose the concept of “Long COVID”. The editorial said, as the Covid-19 pandemic continues, millions of people might be affected by symptoms such as persistent fatigue, breathing difficulties and depression. However, the rehabilitation issue of the patients is ignored due to lack of proven therapies and guidance. “Long COVID” will affect people’s normal life and work, increase the medical and healthcare burdens and exacerbate the economic loss. On January 17, 2020, in the “Diagnosis and Treatment Scheme of COVID-19 (4th Edition of Trial), the medical community of China officially proposed TCM treatment for the Covid-19 patients. However, the current TCM intervention treatment for the Covid-19 patients’ rehabilitation is mainly based on clinical experience, lacking support from high-quality evidence-based medicine research. Academician Zhong Nanshan and Professor Wang Jian have begun to pay attention to the rehabilitation issue of the patients since the outbreak of the pandemic, who have organized five sub-centers nationwide to launch double-blind, random and control studies on the treatment of the patients during rehabilitation period with the Lung Tonifying and Blood Circulation Promoting capsules.
The research was led by the National Respiratory Medical Center and the First Affiliated Hospital of Guangzhou Medical University and conducted by Wuhan Lung Hospital, the People’s Hospital of Xiangzhou District of Xiangyang, the No. 3 Hospital of Baotou, the Central Hospital of Xiangyang and the School of Public Health of Nanjing Medical University, which is thus far reported as the first double-blind, random, control and multi-center clinical research completed among the Covid-19 patients during rehabilitation period. It was found by the researchers that by treating the patients with oral capsules for Lung Tonifying and Blood Circulation Promoting for 90 days, it can promote the absorption of focus of infection at the lung during rehabilitation period, prevent the focus of infection from worsening, increase the patients’ exercise tolerance, ease their symptoms such as fatigue and weakness, and promote their recovery with a good safety performance. The research has provided Level Ib evidence-based medicine evidence for using the aforesaid capsule in the treatment of the patients during rehabilitation. This completed research will increase attention to the rehabilitation of patients and provide high-level evidence-based medicine evidences for using TCM in the treatment of patients during rehabilitation.
The research is recently published in the “Journal of Ethnopharmacology” in the first zone of general family medicine and complementary medicine. The research’s corresponding authors are academician Zhong Nanshan and Professor Wang Jian with the National Respiratory Medical Center and the First Affiliated Hospital of Guangzhou Medical University. The first authors include deputy research fellow Chen Yuqin and Professor Liu Chunli with the National Respiratory Medical Center and the First Affiliated Hospital of Guangzhou Medical University, chief physician Wang Tingping with Wuhan Lung Hospital, chief physician Qi Jingjing with the People’s Hospital of Xiangzhou District, Xiangyang, associate professor Bo Jianling with the School of Public Health of Nanjing Medical University, and Professor Lu Wenju with the SKLRD.
Design and process of the research:
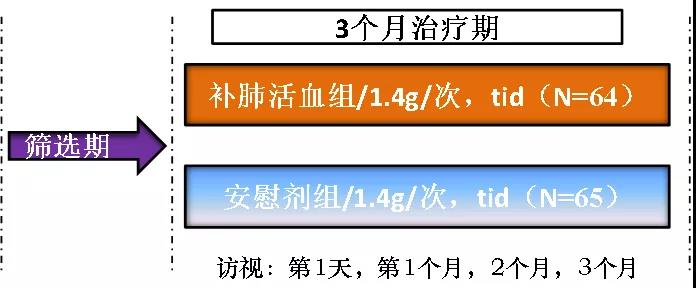
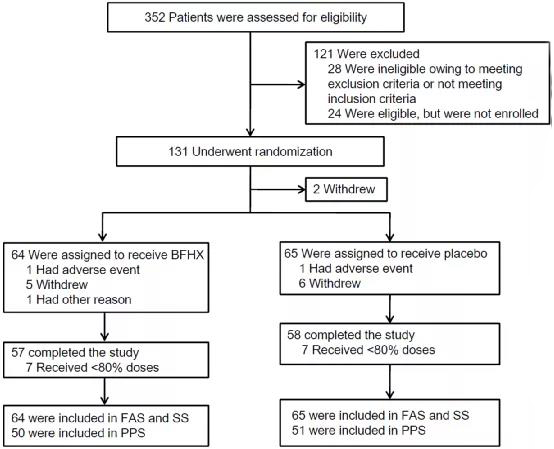
In the research, 131 patients were accessed. According to the research scheme, the selected patients were randomly divided into the treatment group and the placebo group. The patients in the treatment group were offered oral administration of 4 capsules/time, three times a day, for a treatment period of 90 days. The primary indicators include lung CT improvement rate and six-minute walk distance, and the secondary indicators include the score of St. George respiratory questionnaire, the score of TCM syndrome, the fatigue score and the score of Borg breathing difficulty index.
The main research findings include: 1. The said capsule can promote the improvement of lung imaging indicators among the patients.
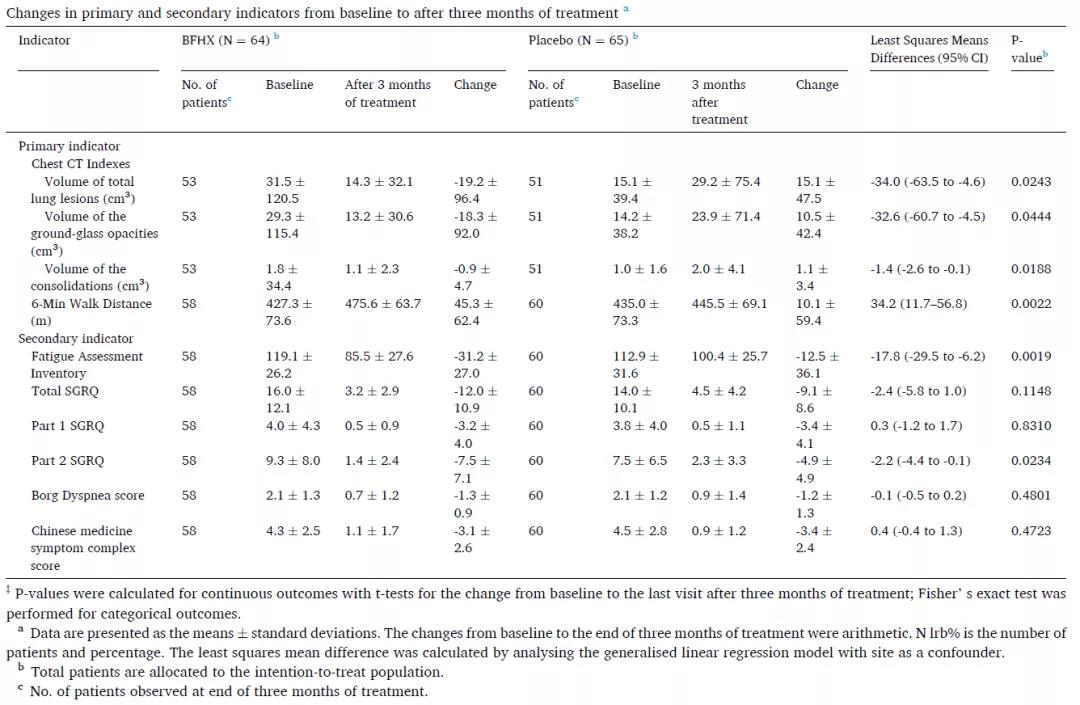
Table 1: Changes of primary and secondary indicators before and after the treatment
After receiving treatment with the capsule for 90 days, the treatment group has witnessed a significance rise in the improvement rate of lung CT than the placebo group, and the size of focus of infection in their lung (ground-glass opacity or consolidation) has been reduced significantly.
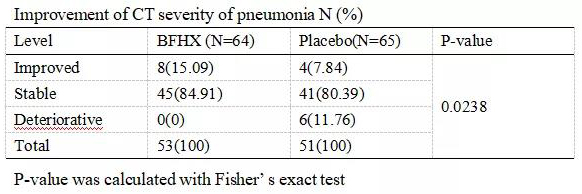
Table 2: The improvement of patients in CT severity of pneumonia. The patients in the treatment group witnessed no deterioration in their lung CT, while 11.76% of the patients in the placebo group showed a worsened CT severity of pneumonia
2. The said capsule can improve the patients’ exercise tolerance during rehabilitation
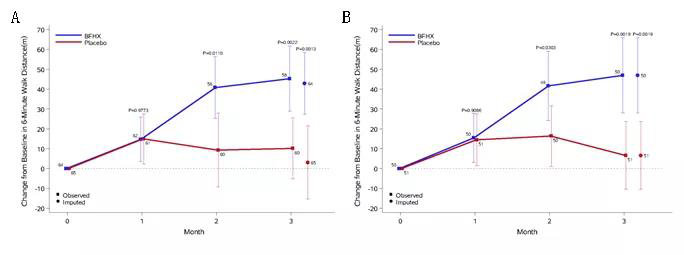
Fig. 3: The differences of the changes between the two groups in terms of six-minute walk distance. After receiving the treatment for three months, the treatment group’s changes in six-minute walk distance are obviously better than the placebo group. A refers to full analysis set (FAS) and B refers to per-protocol set (PPS)
3. The said capsule can ease the fatigue of the patients during rehabilitation
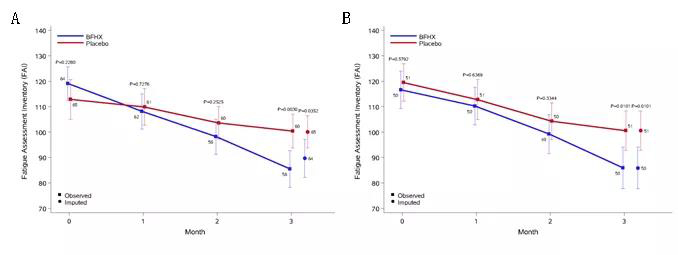
Fig. 4: The differences of fatigue score between the two groups. After receiving the treatment for three months, the treatment group’s fatigue score is obviously better than the placebo group. A means full analysis set (FAS) and B means per-protocol set (PPS)
In addition, adverse events that occurred in the research were mainly light ones. There is no statistical difference between the two groups, and there is no serious adverse events related with the drug studied.
















