The team led by Zhao Jincun collaborated with the British Gene Therapy Consortium for Lung Diseases: Intranasal Lentiviral Vector Mediated Antibody Delivery Confers Reduction of SAR...
2022-03-251334On March 18, 2022, the team led by Zhao Jincun from SKLRD collaborated with the team led by Professor Deborah Gill, the Department of Clinical Medicine, Oxford School of Medicine, to publish a study titled “Intranasal Lentiviral Vector Mediated Antibody Delivery Confers Reduction of SARS-CoV-2 Infection in Elderly and Immunocompromised Mice” in the journal Frontiers in Immunology-Vaccine and Molecular Therapeutics (Figure 1). This proof-of-concept study utilizes the novel replication-defective lentiviral vector, rSIV.F/HN technology platform to successfully develop the first COVID-19 vectored-immunoprophylaxis for the elderly and the immunocompromised population, and has proved its long-term protective efficacy, which can still provide effective protection against novel coronavirus infection 180 days after a single administration.
Unlike traditional vaccines, the team’s technical strategy is to perform intranasal delivery of antibody genes using viral vectors with high affinity to the lungs to mediate immunoprophylaxis, with gene sequences that can synthesize monoclonal antibodies directly in lung cells, which can be secreted into the local environment and eventually circulated throughout the body. The results of the experiment show that this strategy produces efficient and long-term antiviral effects among healthy mice or elderly and immunocompromised mice. This virus-vector-based immunoprophylaxis solution provides a new idea to prevent SARS-CoV-2 and other respiratory viral infections in immunocompromised and aged individuals (especially those with poor response to conventional vaccines).

Figure 1. First page of the article published in the official website of Frontiers in Immunology
Professor Zhao’s team successfully screened broad-spectrum and efficient neutralizing antibody NC0321 from the PBMC of patients recovering from Covid-19 infection, which can neutralize multiple variant strains of novel coronavirus including wild-type, Alpha, Beta, Delta, Eta. Meanwhile, based on the adenovirus-transduced mouse animal model 1, it has demonstrated that NC0321 antibody can effectively prevent and treat mice from SARS-CoV-2 infection and thus boast a potential in clinical application (Figure 2).
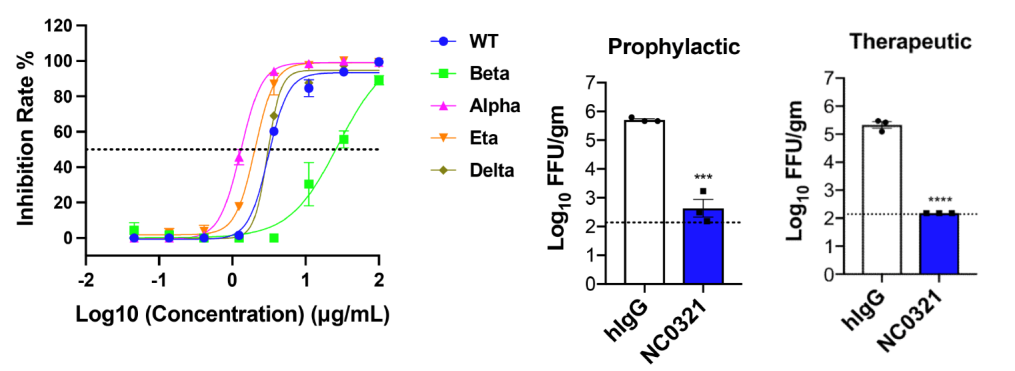
Figure 2. In vitro and in vivo neutralization capability detection of NC0321 antibodies
Professor Deborah Gill’s team has developed a novel replication-defective recombinant lentiviral vector, rSIV.F/HN, based on the simian immunodeficiency virus (SIV). Precise delivery to lung cells and gene transfer efficiency can be achieved by a single intranasal injection or inhalation, and it has the ability to repeat drug administration 2. Using the mature rSIV.F / HN vector suspension production and purification process established earlier, the team prepared and produced a high-titer rSIV.F/HN vector carrying the NC0321 antibody gene or the engineering modified gene fragment. The results in the experiment of mice showed that a single intranasal rSIV.F / HN gene delivery platform expressed the NC0321 antibody. Through in vivo imaging observation, compared with the healthy mice in the experiment both elderly mice and immunodeficient mice could effectively prevent the lung infection (fluorescent display, Figure 3A) from the SARS-CoV-2 adapted strain simulant (pseudovirus model), and there was no gender difference. At the end of the experiment, it confirmed that there were high concentrations of NC0321 antibody expressed in the serum and lungs of each group and showed no difference among the groups (Figure 3B).
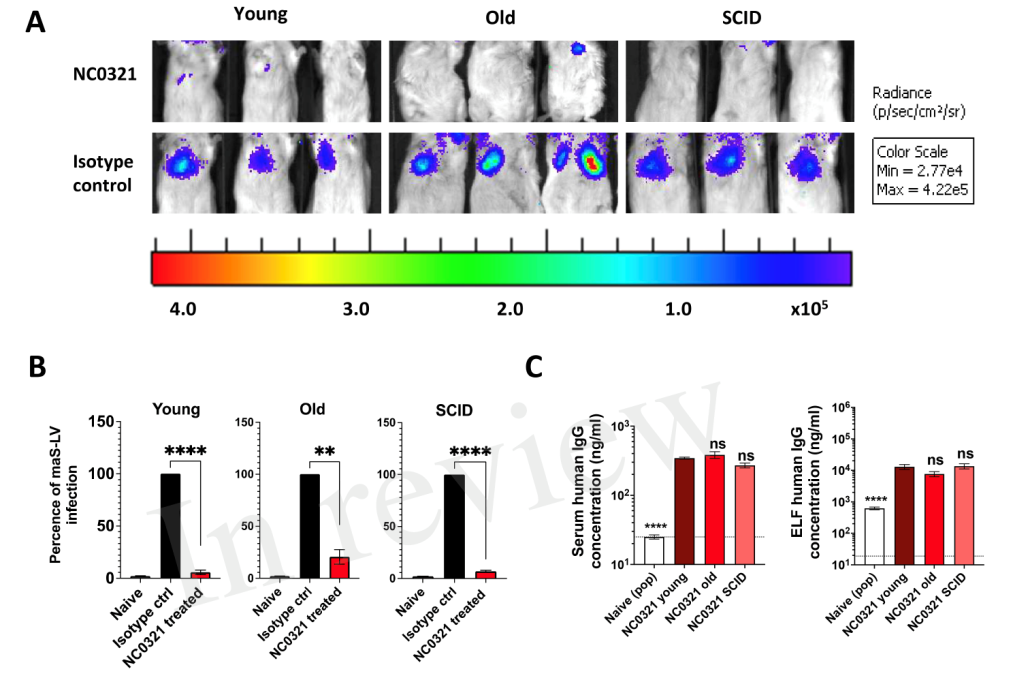
Figure 3. In vivo NC0321 action was detected with the pseudovirus method
Besides, the rSIV. F / HN-NC0321 intranasal transduced mice underwent SARS-CoV-2 live infection on the 30th and 180th of immunoprophylaxis, and showed significantly lower viral titer in the lungs compared with adenovirus-expressing hACE2 mice in the control group (Figure 4). These results suggest that the biologically active monoclonal antibody genes carried by rSIV.F/HN can protect mice at different healthy ages against viral infection, and boast long-term protective effects.
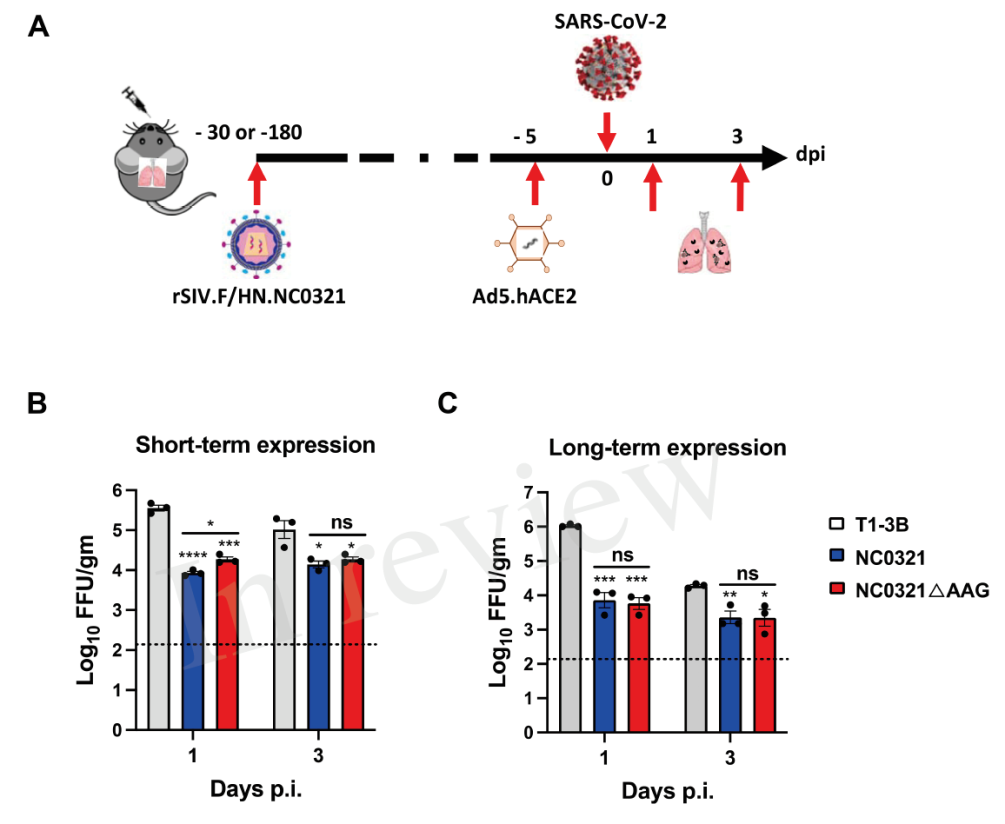
Figure 4. Delivery of the rSIV. F / HN-NC0321 is effective in preventing SARS-CoV-2 infection
The elderly and immunocompromised individuals are often less effective or unable to induce effective antibodies due to their weaker or defective immune response. In this study, the replication-defective recombinant Lentiviral rSIV.F/HN was expressed by intranasal delivery of bio active neutralizing antibodies, which boasts a potential in providing long-term and efficient protection for these populations, which is of certain guiding significance in filling the pandemic prevention loopholes. In the future, it will be provided in the form of nasal spray to protect the recipients from COVID-19 or other respiratory diseases (such as influenza, syncytial virus, etc.). Compared with the antibody treatment with a short validity window, this study using recombinant Lentiviral vector can achieve long-term expression, greatly improve the validity and efficiency of neutralizing antibodies, with protective duration similar to that of vaccines, which can replace antibody sequence according to the virus variant (or a cocktail strategy for multiple antibodies) and allow repeated administration. This strategy is of important reference for the protection of front-line medical workers exposed to the virus environment and the populations with weak immunity.
1.Sun, J. et al. Generation of a Broadly Useful Model for COVID-19 Pathogenesis, Vaccination, and Treatment. Cell 182, 734-743.e735, doi:10.1016/j.cell.2020.06.010 (2020).
2.https://www.respiratorygenetherapy.org.uk/copy-of-news202101
3.https://www.respiratorygenetherapy.org.uk/
















