Associate Professor Wang Tao’s team finds that the intestinal flora aggravates pulmonary hypertension through the synthesis of trimethylamine oxide
2022-03-14965Recently, Associate Professor Wang Tao’s team from the Pulmonary Vascular Disease Group of the SKLRD has published an original research online titled “Gut Microbial Metabolite Trimethylamine N-Oxide Aggravates Pulmonary Hypertension” in the leading respiratory journal American Journal of Respiratory Cell and Molecular Biology (IF: 6.914, Journal of First Area of Chinese Academy of Sciences). It was found that trimethylamine N-oxide (TMAO), a metabolite of intestinal flora, plays an important role in the vascular remodeling in pulmonary hypertension. By inhibiting the production of intestinal TMAO, it can reduce the pulmonary hypertension vascular lesions and reduce the right heart resistance, providing a new idea for the treatment of pulmonary hypertension.
Pulmonary hypertension (pulmonary hypertension, PH) is a fatal disease or clinical syndrome caused by multiple factors and links together and characterized by pulmonary vascular dysfunction, progressive pulmonary vascular remodeling and continuous rising of pulmonary artery pressure. The excessive proliferation and migration of pulmonary artery smooth muscle cells are the main pathological changes of pulmonary vascular remodeling of PH. At present, there are some clinical contraindications for PH treatment drugs, which are ineffective for the treatment of some types of PH caused by lung diseases. Therefore, by exploring the new mechanism of hyperproliferation and migration of pulmonary artery smooth muscle cells in PH and developing new therapeutic targets on this basis, it could provide more options for the treatment of PH.
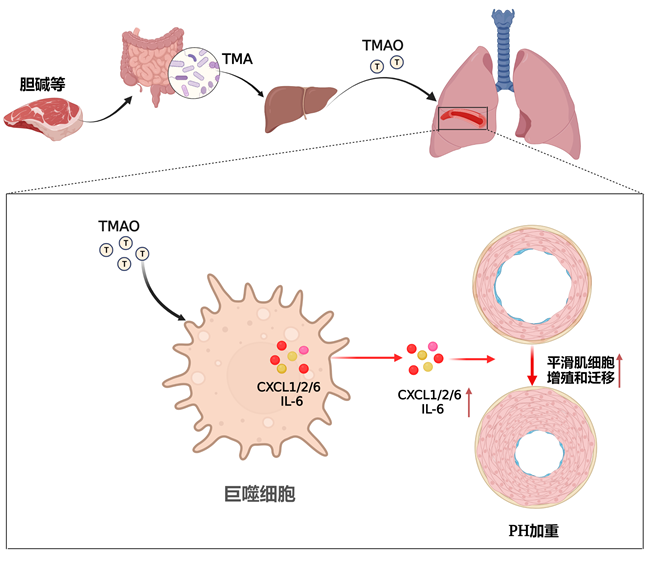
Recent studies show significant differences in intestinal flora components between PH patients and healthy controls, and that intestinal flora transplantation can reduce pulmonary vasculopathy in animal models of PH. TMAO is a metabolite formed by choline and other substances in food after being converted into TMA by the trimethylamine (TMA) synthase unique to the intestinal flora and later further oxidized in the liver. In this process, the intestinal flora is an essential link of TMAO synthesis. Professor Wang’s team found elevated TMAO expression in plasma of monocrotaline-induced PH rats; blood TMAO levels were higher in severe PH patients than in healthy controls and less severe PH patients. Inhibition of TMAO synthesis by intestinal flora of PH animals can effectively reduce the right ventricular systolic blood pressure and reduce the pulmonary vascular remodeling. Mechanism studies found that TMAO directly stimulated pulmonary artery smooth muscle cells and later had no effect on their proliferation and migration, while the results of RNAseq (transcriptome sequencing) suggested that the main pathways regulated by TMAO were concentrated on inflammatory factor-related pathways. Further experiments showed that TMAO can increase the synthesis and secretion of macrophages such as CXCL1, CXCL2, CXCL6 or (and) IL6, and that applying the culture medium after TMAO-stimulated macrophages acted on smooth muscle cells can cause excessive proliferation and migration of smooth muscle cells. This study is the first one to reveal the role of TMAO in PH, indicating that reducing TMAO synthesis through the inhibition of intestinal flora and daily dietary interventions may be a potential treatment for PH.

After the above results were published in Am J Respir Cell Mol Biol, Professor Micheala A Aldred of Indiana University wrote an editorial for this article, arguing that our study was the first one to reveal the action mechanism of intestinal flora on PH at the molecule level, and highly praised that the data obtained by applicants would trigger new thinking on intestinal flora and PH research (the data thus far definitely give us food for thought).
















