The Team Led by Academician Zhong Nanshan and Professor Wang Jian Revealing the Molecular Mechanism behind the SARS-CoV-2 Spike Protein’s Impairing Pulmonary Vascular Endothelial ...
2023-07-192550Since the end of 2019, the global outbreak of COVID-19 pandemic caused by the novel coronavirus SARS-CoV-2 infection has posed a huge threat to human health. Under the current trend of long-term coexistence with the virus, the prevention and management of the sequelae of COVID-19 have become the core concerns of society. SARS-CoV-2 infection induces severe endotheliopathy in patients, which is characterized by substantial endothelial cell damage and microthrombus aggregation in the pulmonary vasculature.Studies have shown that the interaction of SARS-CoV-2 spike protein with endothelial cells and the subsequently induced micro-environment changes are sufficient to damage the pulmonary vascular endothelium and drive the endotheliopathy and endothelial dysfunction, though the specific molecular mechanism is to be further studied and confirmed.
On July 14, 2023, The State Key Laboratory of Respiratory Diseases of the Guangzhou Medical University, the team led by Academician Zhong Nanshan and Professor Wang Jian with the Guangzhou Laboratory published a research article online in the journal Signal Transduction and Targeted Therapy under Nature (Top Journal of District 1 Medicine of Chinese Academy of Sciences, IF=39.3), entitled “SARS-CoV-2 Spike Protein Receptor-Binding Domain Perturbates Intracellular Calcium Homeostasis and Impairs Pulmonary Vascular Endothelial Cells”. This study revealed that the abnormal expression and activation of store-operated calcium channel (SOCC) and mechanosensitive ion channel Piezo1 play an important role in pulmonary vascular endothelial injury and vascular remodeling induced by SARS-CoV-2 spike protein, providing a new mechanism and a new target for the prevention and treatment of pulmonary vascular diseases caused by SARS-CoV-2. Academician Zhong Nanshan, Professor Wang Jian and Professor Yang Kai with the SKLRD are the corresponding authors of the article, Professor Yang Kai, doctoral candidates Liu Shiyun and Professors Yan Han and Lu Wenju are the first authors.
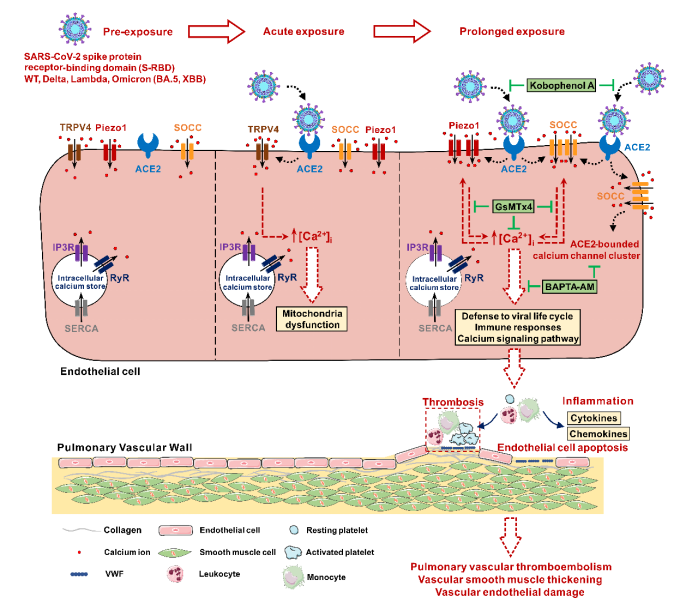
First, in the human pulmonary arterial endothelial cells (PAECs) cultured in vivo, acute exposure (1, 2h) to SARS-CoV-2 spike protein receptor-binding domain (S-RBD) enhances intracellular calcium concentration ([Ca2+]i) via triggering extracellular calcium influx through TRPV4; Prolonged exposure (24, 72h) to S-RBD elevates [Ca2+]I via enhancing Piezo1 and SOCC activity in human PAECs.
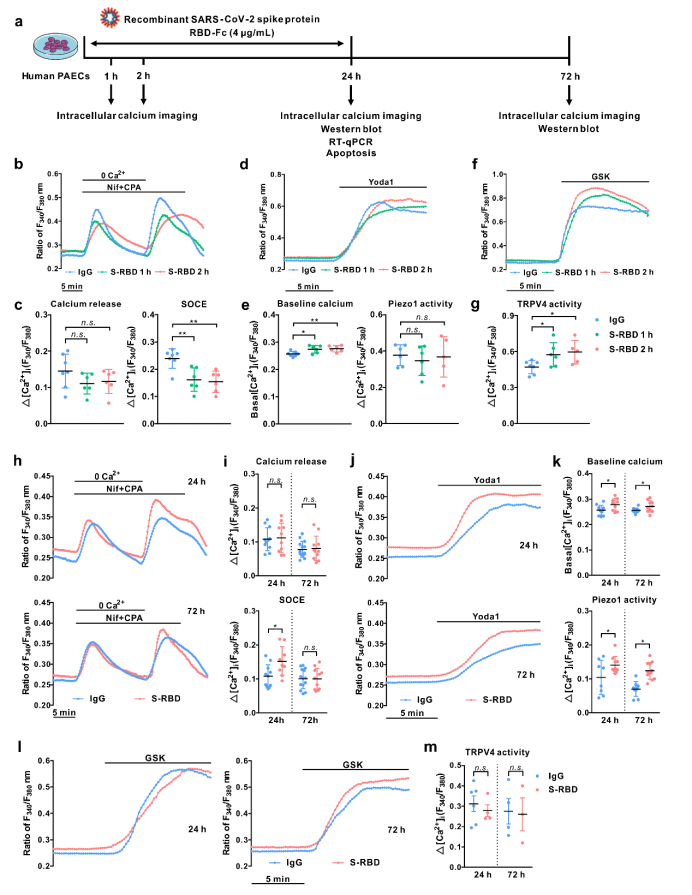
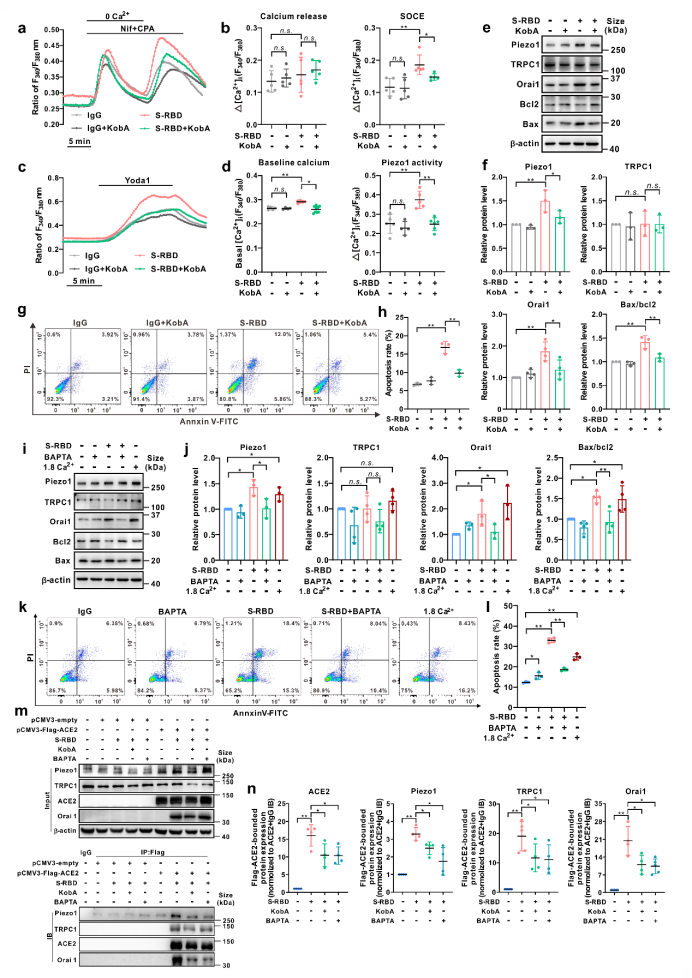
Secondly, by analyzing the protein expression of related calcium channel components, such as Piezo1, TRPV4, and SOCC (three major components including STIM1, TRPC1 and Orai1), we discovered that prolong exposure to S-RBD (24, 72h) may significantly upregulate the protein expression of Piezo1 and Orai1. Meanwhile, specific knockdown of Piezo1, Orai1 or STIM1 using siRNA may significantly inhibit S-RBD induced intracellular calcium upregulation and excessive apoptosis of cells.
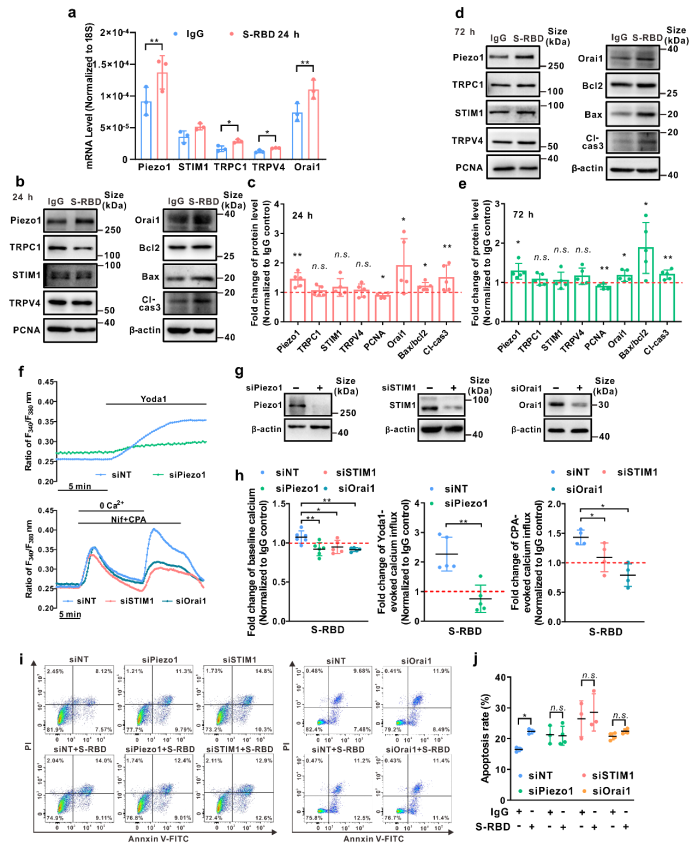
The specific blocking of the interaction between S-RBD and host-cell main receptor ACE2 with the inhibitor Kobophenol A (KobA) or the intracellular calcium chelating agent BAPTA-AM (BAPTA) has confirmed that S-RBD-induced PAECs apoptosis and the up-regulation and activation of Piezo1 and SOCC calcium channels are dependent on the interaction of S-RBD-ACE2 and the presence of intracellular calcium ions. S-RBD exposure has been confirmed by immunoprecipitation experiments in HEK 293T cells with the heterogeneous expression of ACE2 that it may induce the formation of polymers in calcium channels including ACE2 and Piezo1, Orai1, TRPC1 by binding with the host-cell ACE2, and promote the up-regulation and activation of calcium channels such as Piezo1 and SOCC, resulting in cell apoptosis. However, pretreatment with either KobA or BAPTA may significantly inhibit the progression of this process.
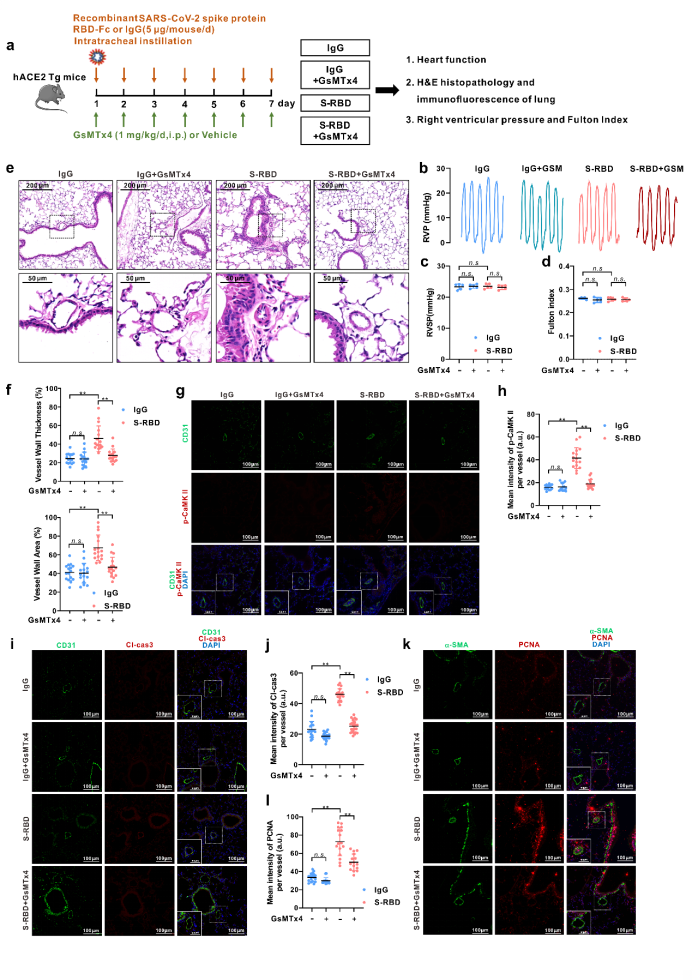
To further elucidate the impacts of S-RBD on pulmonary vessels, an animal model was developed using humanized ACE2 transgenic mice (hACE2 Tg) infused with S-RBD recombinant protein via continuous instillation with trachea cannula for 7 days in succession. On the one hand, compared with the IgG control group, the S-RBD may induce significant thickening and remodeling of pulmonary small vessels, accompanied by excessive apoptosis of pulmonary vascular endothelium and excessive proliferation of smooth muscle; on the other hand, by inhibiting the activity of Piezo1 and SOCC with GsMTx4, it may significantly relieve pulmonary vascular remodeling and endothelial damage induced by S-RBD.
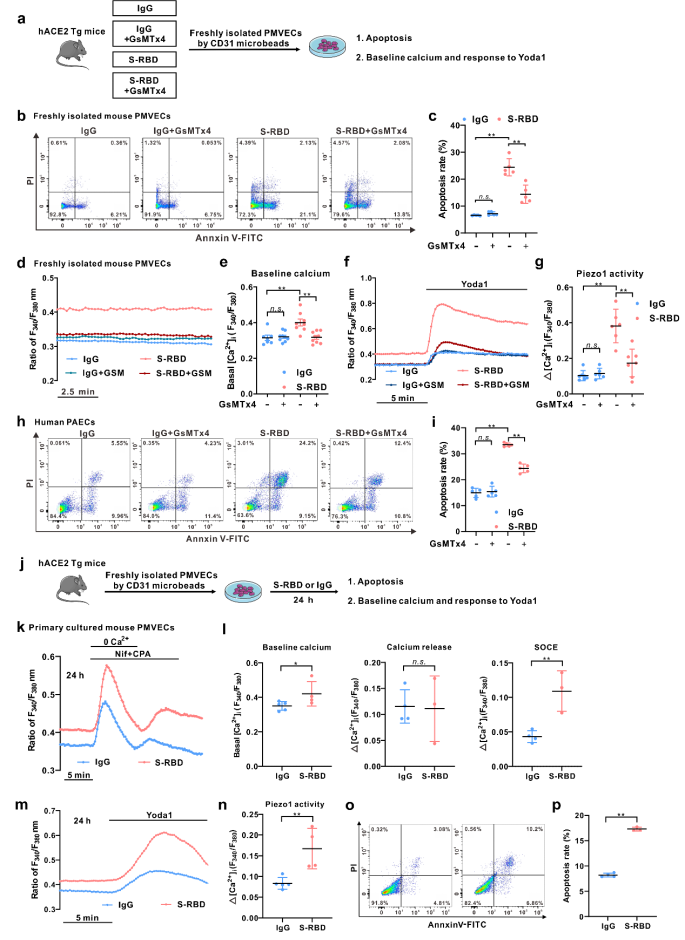
Next, in order to better assess the phenotype and function of pulmonary vascular endothelial cells of mice in each group, the pulmonary microvascular endothelial cells (PMVECs) of each group were freshly isolated and cultured with magnetic activated cell sorting technology. The results showed that, on the one hand, freshly isolated PMVECs in the S-RBD-treated mice showed a significant rise of intracellular calcium and apoptosis levels, while the cells of related phenotypes sorted in the GsMTx4 treatment group were significantly improved. On the other hand, in PMVECs isolated and cultured from mice with normal hACE2Tg, prolong exposure to S-RBD can also induce significantly elevated levels of intracellular calcium and apoptosis.
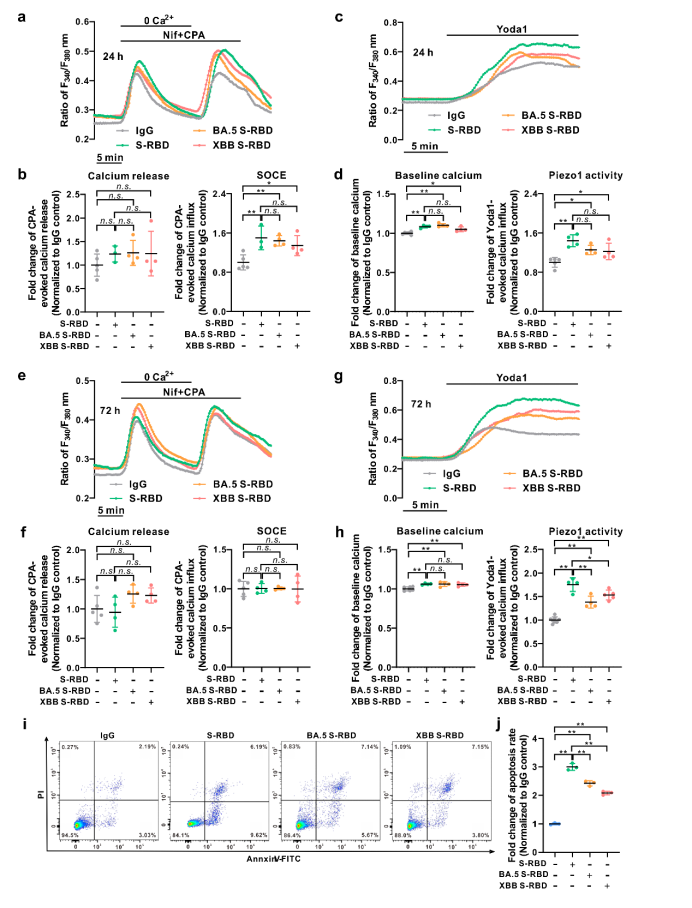
In addition, the study also compared S-RBD of different mutant strains, such as the impacts of Omicron (BA.5.2 and XBB) on the intracellular calcium homeostasis regulation and apoptosis in human PAECs. It was discovered that compared with the original strain’s S-RBD, the S-RBD of Omicron mutant strain (BA.5.2 and XBB) induced significantly lower apoptosis, with basal calcium at a comparable level, and the activity of SOCE and Piezo1 channels declined slightly.
This study reported for the first time that S-RBD exposure can promote the rise of calcium ion concentration in pulmonary vascular endothelial cells by upregulating and activating calcium channels such as Piezo1 and SOCC, thereby inducing excessive apoptosis of PAECs and abnormal pulmonary vascular remodeling. Targeted inhibition of the host-cell ACE2-Piezo1/SOCC-[Ca2+]i signaling axis may be a new effective treatment against pulmonary vascular injury induced by S-RBD (even SARS-CoV-2), providing new ideas and new strategies for the prevention and control of pulmonary vascular complications of long Covid-19.