The Team Led by Professor Chen Ling Achieves a Progress in the Research of Intranasal Vaccination for Omicron
2023-04-191626Some variants of Novel Coronavirus (Omicron) have been spreading wide around the world, which has significantly reduced the effect of intramuscular COVID-19 vaccine designed for the original strain. It is urgent for us to develop some new vaccines and develop new immune delivery strategies. Omicron infection mainly occurs in the nasopharynx, so vaccines that can effectively induce a mucosal immune responses in the upper respiratory tract may prevent the infection and transmission of Novel Coronavirus.
Recently, the team led by Professor Chen Ling has made a new progress in this field in collaboration with other teams. Their research findings have been published in the international academic journal Signal Transduction and Targeted Therapy with the title of “Intranasal booster using an Omicron vaccine confers broad mucosal and systemic immunity against SARS-CoV-2 variants”.
The Ad5-S-Omicron (NB2155) vaccine was jointly developed by the SKLRD, the Guangzhou Laboratory and Guangzhou Enbao Biomedical Technology Co., Ltd. The research team developed the recombinant human replication-defective adenovirus vector vaccine Ad5-S-WT (NB2001) for the original strain of Novel Coronavirus in early stage reported for the first time that intranasal vaccination could induce respiratory tract mucosal and systemic immune responses in macaques, and revealed that it could better protect macaques against the infection of the original strain than intramuscular vaccine (Nature Communications 2020, 11:4207). After Omicron became the Variant of Concern (VOC) it took only 24 days for the team to develop recombinant human adenovirus vector vaccine Ad5-S-Omicron (NB2155). The guinea pigs with intranasal vaccination not only could induce specific serum neutralizing antibodies and systemic T cell responses, but also could induce the formation of specific respiratory mucosa IgA and lung-resident T cell responses, while the guinea pigs with intramuscular vaccine could not induce respiratory mucosal immune responses (Fig. 1).
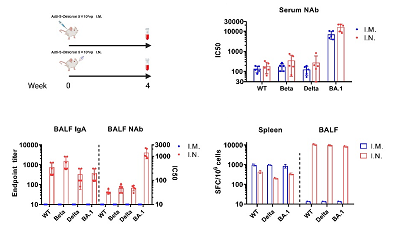
Figure 1. Comparison of the immunogenicity of Ad5-S-Omicron in guinea pigs with intranasal vaccination and intramuscular vaccination
It is noteworthy that among the guinea pigs, intranasal vaccination on the basis of the inactivated vaccine was intramuscularly injected could induce the formation of mucosal IgA and serum neutralizing antibodies with a broad spectrum of neutralizing activity against multiple variants of Omicron (Omicron BA.1, BA.2, BA.5, BA.2.75 and BF.7), the original strain, Beta, and Delta, which also had certain neutralizing activity against the currently prevailing strains XBB, BQ.1 and BQ.1.1 with a strong immune escape ability. To verify the protective effect of the intranasal Ad5-S-Omicron vaccine on the basis of the inactivated vaccine, the researchers used Omicron BA.2.3 Virus on immunized guinea pigs, which showed the best protection effect (Fig. 2) compared with those guinea pigs not vaccinated or without receiving the inactivated vaccine (but only receiving intranasal Ad5-S-Omicron vaccine). In addition, five volunteers who had previously received the inactivated vaccine, after receiving the intranasal vaccine, could significantly induce secretory IgA antibodies in their nasal mucosa against at least ten variants of Novel Coronavirus, including Omicron BA.1, BA.2, BA.3, BA.1.1, BA.1+L452R/BA.5 like and the IHU/B.1640.2, original strain, Alpha, Beta and Delta. Interestingly, when nasal wash (NLF) of volunteers with intranasal vaccine was passed onto the guinea pigs, it could also allow them to defend against the infection of BA. 1.1 (Fig. 3), which suggested that secretory IgA in the nasal mucosa had the ability to prevent Omicron infection. This study revealed that the intranasal Ad5-S-Omicron vaccine on the basis of the inactivated vaccine against the original strain could establish broad-spectrum mucosal and systemic immune responses at the upper respiratory tract against different Omicron variants, which is of great significance for effectively preventing infection and blocking the transmission of Omicron variants.

Figure 2. Intranasal Ad5-S-Omicron vaccine on the basis of the inactivated vaccine for original strain can induce broad-spectrum secretory IgA and systemic immunity in the respiratory mucosa
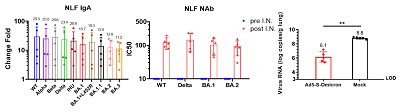
Figure 3. After the intranasal vaccination, the secretory IgA significantly increased among the volunteers, which could identify multiple variants and had neutralizing activity. The nasal wash applied on the guinea pigs could defend against Omicron
Post-doctor Wang Qian (now an associate research fellow with Guangzhou Laboratory), Yang Chenchen with Guangzhou Enbao Biomedical Technology Co., Ltd., Professor Yin Li with Guangzhou Health Institute of Chinese Academy of Sciences, Professor Sun Jing, and Dr. Wang Wei of the Bioland Lab are co-first authors of this paper. Professor Chen Ling, Professor Zhao Jincun and Associate research fellow Li Pingchao with Guangzhou Health Institute of Chinese Academy of Sciences are co-corresponding authors of the paper. Academician Zhong Nanshan has offered strong support and guidance. The research has been supported by Guangzhou Laboratory, and funded by National Natural Science Foundation of China, Youth Innovation Promotion Association of Chinese Academy of Sciences, the Department of Science and Technology of Guangdong, Guangzhou Municipal Science and Technology Bureau, etc.

Figure 4. Sequential booster immunization with Ad5-S-Omicron (NB2155) vaccine on the basis of the vaccination for original strain can induce broad-spectrum mucosal immunity against Omicron infection
Link of the paper:https://www.nature.com/articles/s41392-023-01423-6
















